Global insights for smarter market access strategies
MAESTrO brings together global market access submission and outcome insights, to help you introduce new medical technologies more efficiently.
Play video Newsletter signup Download brochure Contact us

Intelligent market access
MAESTrO integrates years of market access submissions and outcomes from all the world’s major health technology assessment (HTA) agencies.
Global perspective
We cover all major HTA markets to provide global, regional and local market access intelligence.
Expert delivery
Our data and interface are constantly fine-tuned by highly experienced industry analysts.
One-stop service
We gather and organise all the essential data in one place, so our solution is all you need.
You’ll be in good company
Real benefits
By combining, analysing and correlating complex data into a one-stop resource, MAESTrO can help you mitigate the costs, delays and risks of medical technology development. Use our data, analysis and platform to:
Gain valuable insights
into successful submission strategies, and benchmark outcomes against competitors.
Compare decisions
between regions and agencies, and assess the effect of past or planned policies.
Compare technologies
for their proposed benefits and potential to be subsidised by the local authority.
Analyse opportunities
based on past successes, emerging technologies, and potential competition.
Global coverage
MAESTrO offers you simple access to a world of correlated submission and outcome data.
-
Agencies18
-
Countries33
-
Entries47,507
-
Technologies13,959
-
Diseases4,446
-
Companies5,295
Powerful, flexible filters
With MAESTrO your market access and investment decisions can be better informed. Our advanced search and data filters make it easy to find, sort and analyse information that once took months to compile. Interrogate global and local market access data by:
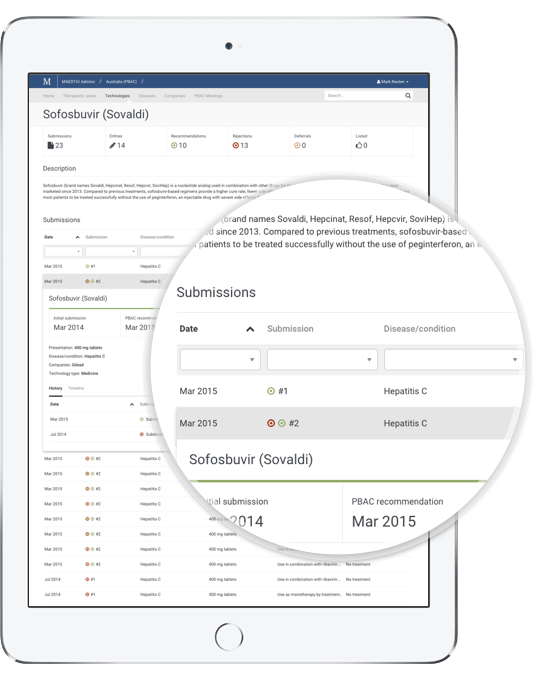
- Technology type
- Technology
- Disease
- Patient population
- Therapeutic area
- Company
- Outcome
- Agency
- Technology category
- Policy issue
- and more...
Deeper analysis
MAESTrO also provides convenient unique access to several facets of the market access record, which can add essential depth to your analysis.
Reason for rejection
Know the reason given by an HTA for rejecting each unsuccessful submission

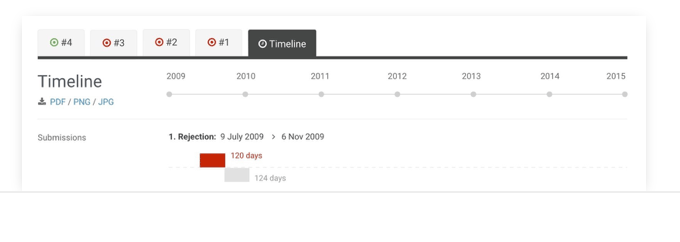
Timeline
See the duration of entire submission processes, to better analyse company and HTA performance.
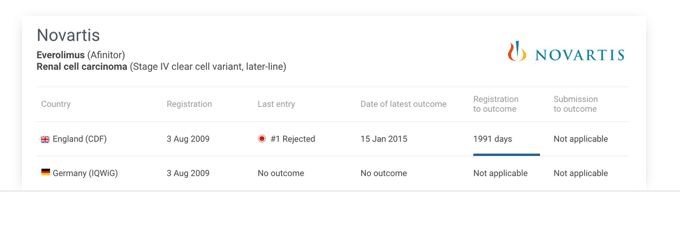
International
Compare a new technology’s submission progress across multiple international HTAs.
Full submission histories
Have automatic access to all resubmissions when viewing the initial submission result.